J.J. Thomson (1856 - 1940)
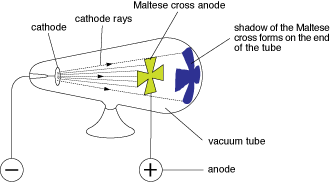
Thomson discovered the electron. He used a cathode ray tube and pumped all the air out of it. He had two pieces of metal in side of the tube that be connected to a power source. As a result, a ray shot from one piece of metal across the tube. He took two metal plates and placed them on either side of the tube and turned on the electricity. The top plate was positive and the bottom plate was negative. Thomson knows that opposites attract. When the cathode ray bended up towards the positive charge he concluded that the cathode ray must be made of stuff that is negatively charged. He also uses a magnet to test what his experiment because he knows that if something has an negative charge and it is moving the magnet will push the ray in a particular way. Thomson surrounds the cathode ray tube by the magnet and he sees that the cathode ray bends in the other direction. This shows that whatever makes up the cathode ray is nagatively charged. Thomson concludes that atoms have tiny, negatively charged particles inside them called electrons. To make sure his data was accurate, Thomson used all different types of metal but his result was the same. All different types of metal give off cathode rays. Also, he concluded that all cathode rays particles are identical.
Thomson found out that particles that make up cathode rays are 1,000 times smaller than a hydrogen atom.
The name of his model was the Plum Pudding Model. When he placed the magnet the positive plate attracted while the negative plate repelled the ray which means that there is a negative charge since it attracted the positive metal and repelled the negative plate. Thomson believed that the electrons were stuff in positive stuff in order to balance out the electron, giving the atom a neutral charge. He knew the ray had negative particles but the atom had to be overall neutral so something positive had to exist.